QUDT.org is a 501(c)(3) public charity nonprofit organization founded to provide semantic specifications for units of measure, quantity kind, dimensions and data types. QUDT is an advocate for the development and implementation of standards to quantify data expressed in RDF and JSON. Our mission is to improve interoperability of data and the specification of information structures through industry standards for Units of Measure, Quantity Kinds, Dimensions and Data Types.
QUDT.org operates thanks to volunteers, but we do have infrastructural costs of course. If you value the QUDT ontologies as a resource available to all, please consider a DONATION of any amount (tax deductible in the USA). That will allow us to keep going and growing.
Why QUDT.org
QUDT.org exists to make the QUDT Ontologies, derived models and vocabularies available to the public. Originally, QUDT models were developed for the NASA Exploration Initiatives Ontology Models (NExIOM) project, a Constellation Program initiative at the AMES Research Center (ARC).
Use Cases Benefitting From QUDT
QUDT is more than a set of vocabularies representing the various quantity and unit standards. It can be used to solve problems. Some of the associated use cases are itemized below:
- Conversion between single and complex unit types
- Dimensional analysis of equations
- Finding out equivalent units in different systems of units
- Finding out equivalent quantity kinds in different systems of quantities
Current Activities
Please see our Announcements Page on GitHub
QUDT BaseURIs
The DOI reference for citations of QUDT is: https://doi.org/10.25504/FAIRsharing.d3pqw7
QUDT is comprised of several linked ontologies. The core design pattern of the QUDT ontology is shown here:
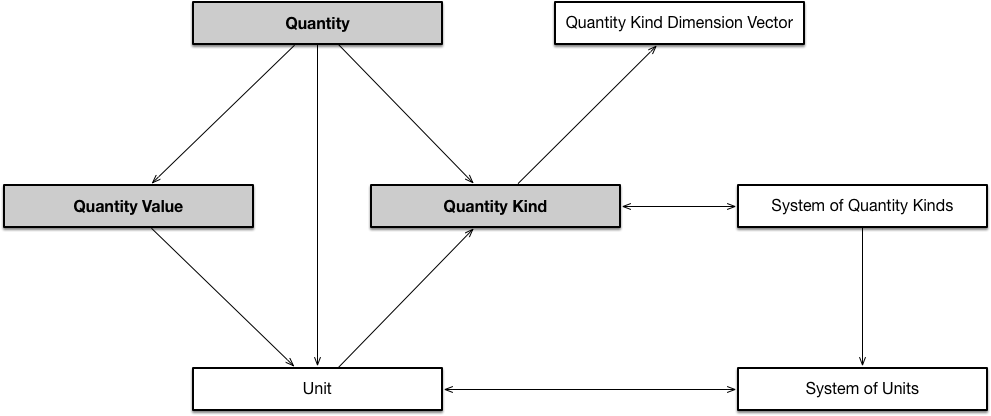
The table below provides the Base URIs for these ontologies.
| Ontology | Base URI |
|---|---|
| Main QUDT Ontology | http://qudt.org/2.1/schema/qudt |
| QUDT Datatype Ontology | http://qudt.org/2.1/schema/datatype |
| QUDT Units Vocabulary | http://qudt.org/2.1/vocab/unit |
| QUDT QuantityKinds Vocabulary | http://qudt.org/2.1/vocab/quantitykind |
| QUDT DimensionVectors Vocabulary | http://qudt.org/2.1/vocab/dimensionvector |
| QUDT Physical Constants Vocabulary | http://qudt.org/2.1/vocab/constant |
| QUDT Systems of Units Vocabulary | http://qudt.org/2.1/vocab/sou |
| QUDT Systems of Quantity Kinds Vocabulary | http://qudt.org/2.1/vocab/soqk |
Governance
QUDT is governed by a board of directors from different organizations.
Last Updated March 1, 2022

This work is licensed under a Creative
Commons Attribution 4.0 International License.
